SARS COV 2 ISOLATION PROTOCOL CONTROL EXPERIMENTS
Vero Cell Line
For the first Experiments released it seems as if Substack Shadow-banned or seriously limited the reach of the results. Despite it being the pinned thread for almost a year and having 500k views on a Twitter thread, Substack claims this under performed by less than 50% all my other articles on here.
Please can you consider Reposting/Cross Posting these latest results so that it beats the restrictions. Many Thanks. Jamie
All Credit goes to Albert .C. Mathews for writing the protocol, financing and managing the experiments. This is my interpretation of the results and observations noted by the Lab technician whom conducted the experiments. There were No Conflicts of Interest.
ABSTRACT
The aim of this experiment was to conduct, document and image a true Negative Control of the Cell Culture Isolation Protocol for Sars Cov 2 using the Vero E6 Cell Line. The Cell Culture is the Gold Standard procedure for isolating viruses and is considered the only method to characterize a new viral strain isolate to be able to use as a benchmark for Immunological and Molecular Test calibration. Without a pure and isolated Biological particle to characterize it renders any subsequent Assay based off of this benchmark as Invalid.
The protocols for Sars Cov 2 Isolation as stipulated by the European Center For Disease Control : Standard laboratory protocols for Sars Cov 2 characterization demonstrate that on Infection of the culture the nutrient medium to feed the Cell Line may be decreased from standard 10% Fetal Bovine Serum Growth medium to a starvation medium of 2%. In our preliminary experiments we comprehensively demonstrated that this lowering of FBS concentration caused CPE morphology in uninfected cultures as well as generated exact particles cross referenced with CDC and NIH images of Sars Cov 2, Measles and HIV in Transmission Electron Microscopy.
In practice, where documented, all Sars Cov 2 Isolations chose to reduce this infection medium down to 2 or even 1% FBS. The Mock Infections claimed in some of these papers ( As alot do not even run one) to operate as a Negative Control almost never document the reagents and their concentrations used. Therefore the aims of these experiments is to conduct these true Negative Controls documenting the exact changes in reagent concentrations run in the Sars Cov 2 Isolations.
METHOD
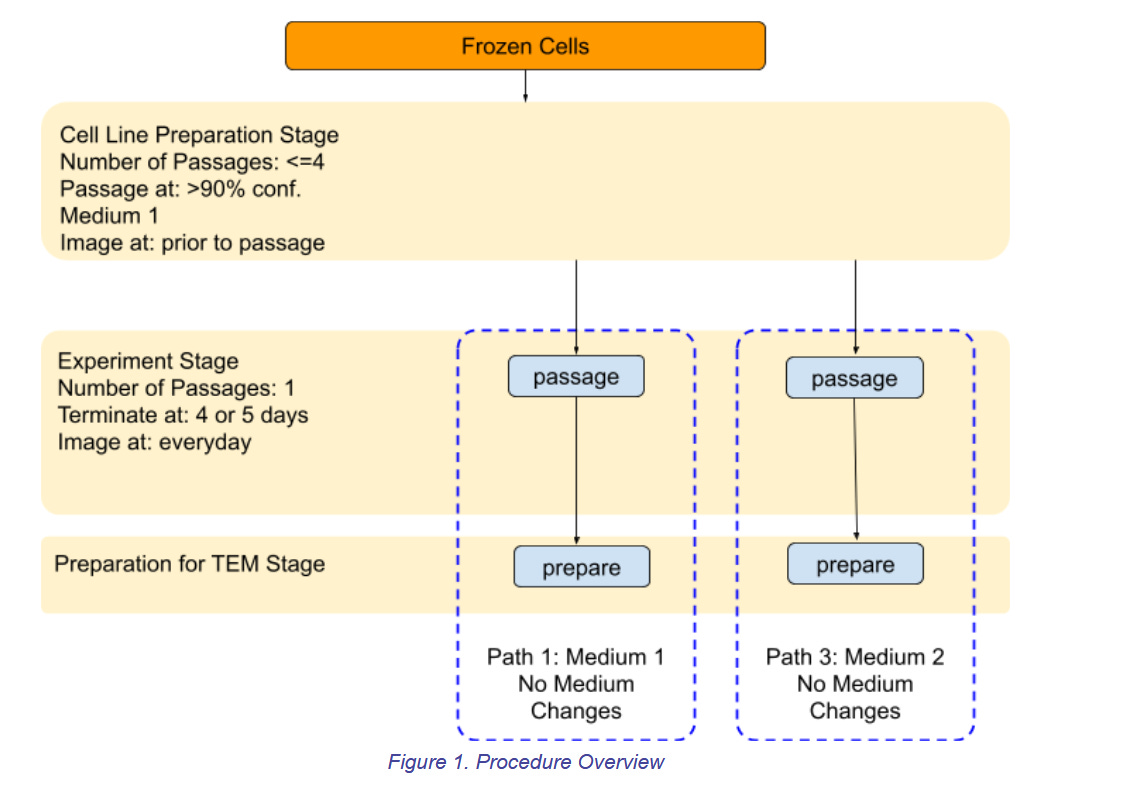
The diagram illustrates the experiment procedure. There are three (3) stages, two (2) cell cultures paths, and two (2) culture mediums.
Detailed Procedure
As shown, cells are thawed, and passaged for the “Cell Line Preparation Stage”. Then the “Experiment Stage” is where the experiment splits into two paths. One passage is performed for the two paths where path 1 uses growth medium 1, and path 2 uses growth medium 2. There is no medium change during this passage of the Experimental Stage. After the experiment stage, the “Preparation for TEM Stage” begins, in which the cell samples are prepared for transport to the TEM lab. Finally the samples are shipped to the TEM lab for imaging.
Subculturing Procedure
By default, subculturing shall follow the ATCC recommended procedure copied here.
Figure 2. Copied Subculturing Procedure
The above procedure is copied from:
● REF2 > Detailed Product Information > Handling Information > Subculturing procedure
Any missing details from the above procedure shall be filled by BASIC PROTOCOL 2 of REF1. Default seeding density is 1-3x10,000 cells/cm2 per REF3. Any deviation shall be subject to approval.
Cell Line Preparation Stage
Per REF1, “After recovery from frozen stock, Vero cells usually take 2-3 passages to reach their regular growth rate”. This stage is intended to achieve this goal. By default, this stage shall follow the ATCC recommended procedure copied here for the first passage after thawing cells.
Figure 3. Copied Thawing Procedure
The above procedure is copied from:
● REF2 > Detailed Product Information > Handling Information > Handling procedure
Any missing details from the above procedure shall be filled by BASIC PROTOCOL 1 of REF1. Subsequent passages required to achieve the goal shall follow the subculturing procedure described above.
Experiment Stage
Cell cultures were split into two paths. Path 1 utilized Medium 1, and Path 2 utilized Medium 2. A single passage was conducted during the experimental stage, lasting five days.
TEM stage: culture samples were prepared as follows:
A. Sample fixing:
1. Discard the medium and immediately add 1.5% or 2.5% glutaraldehyde fixative (pH 7.0) to cover the cells.
2. Scrape and collect the cells into a centrifuge tube.
3. Spin down the cells, discard the supernatant.
4. Add fixative mixed 1:1 with medium and gently pellet.
5. Leave at room temperature (RT) for at least 1 hr.
6. Store at 4°C and ship with ice packs.
B. Sample preparation:
1. Cells were fixed for at least 2 hours at RT in the above fixative. They were then washed in 0.1M cacodylate buffer and postfixed with 1% Osmiumtetroxide (OsO4)/1.5% Potassiumferrocyanide (KFeCN6) for 1 hour. This was followed by two washes in water, one wash in Maleate buffer (MB), and incubation in 1% uranyl acetate in MB for 1 hour, before two final washes in water and subsequent dehydration through graded alcohol solutions (10 min each; 50%, 70%, 90%, 2x10 min 100%).
2. Samples were then immersed in propylene oxide for 1 hour and infiltrated overnight (ON) in a 1:1 mixture of propylene oxide and TAAB Epon (TAAB Laboratories Equipment Ltd, https://taab.co.uk).
3. The following day, samples were embedded in TAAB Epon and polymerized at 60◦C for 48 hours.
4. Ultrathin sections (approximately 80 nm) were cut using a Reichert UltracutS microtome, collected onto copper grids, and stained with lead citrate.
Materials
Vero Cells
ATCC-CRL-1586 [2]
Medium 1
• Eagles Minimum Essential Medium [4]
• 1x Antibiotic-Antimycotic
• 10% heat-inactivated FBS
Medium 2
• Eagles Minimum Essential Medium [4]
• 1x Antibiotic-Antimycotic
• 2% heat-inactivated FBS
Antibiotic-Antimycotic
1x Antibiotic-Antimycotic’ refers to the following final concentrations in the growth
medium:
• penicillin 100 U/ml
• streptomycin 0.1 mg/ml
• amphotericin-B 0.25 µg/ml
Sample Fixative
Cell samples were fixed usng the folloiwing fixtaive before being shipped to TEM lab. 2.5% glutaraldehyde, 1.25% paraformaldehyde, and 0.03% picric acid in 0.1 M sodium cacodylate buffer (pH 7.4).
References
[1] Ammerman NC, Beier-Sexton M, Azad AF. Growth and maintenance of Vero cell lines. Current Protocols in Microbiology. 2008 Nov; Appendix 4:Appendix 4E. doi:10.1002/9780471729259.mca04es11. PMID: 19016439; PMCID: PMC2657228.
[2] American Type Culture Collection, "VERO C1008 [Vero 76, clone E6, Vero E6] Product Page," https://www.atcc.org/products/crl-1586, accessed July 29, 2025.
[3] MilliporeSigma, "Vero C1008 [Vero 76, clone E6, Vero E6] Product Page," https://www.sigmaaldrich.com/CA/en/product/sigma/cb_85020206, accessed July 29, 2025.
[4] American Type Culture Collection, "Eagle’s Minimum Essential Medium (EMEM) Product Page," https://www.atcc.org/products/30-2003, accessed July 29, 2025.
RESULTS
PATH 1 (10%FBS/Negative Control)
Day 1:
Day 2:
DAY 3:
Day 4:
Day 5:
PATH 2 (2% FBS/Test)
Day 1:
Day 2:
Day 3:
Day 4:
Day 5:
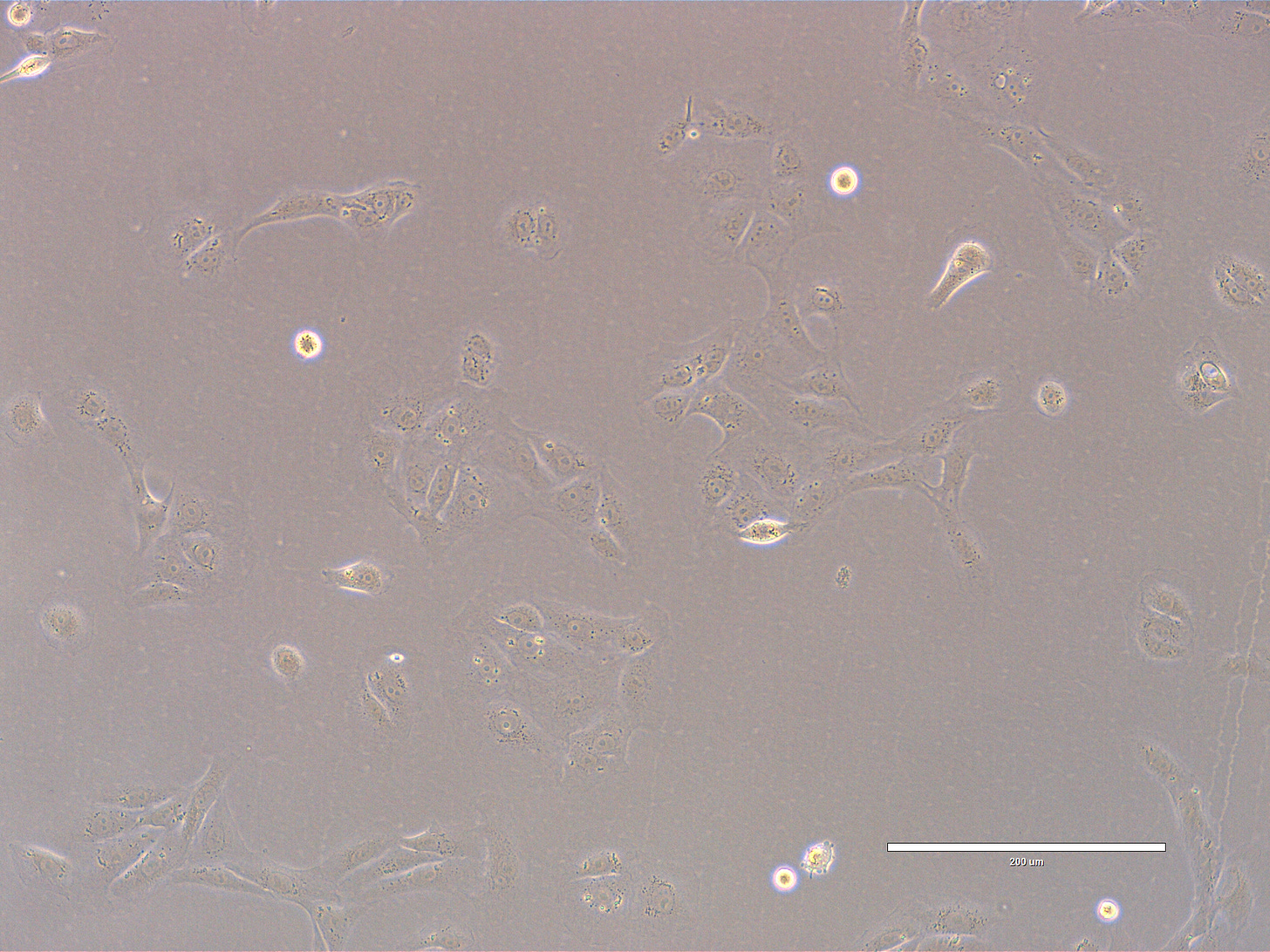
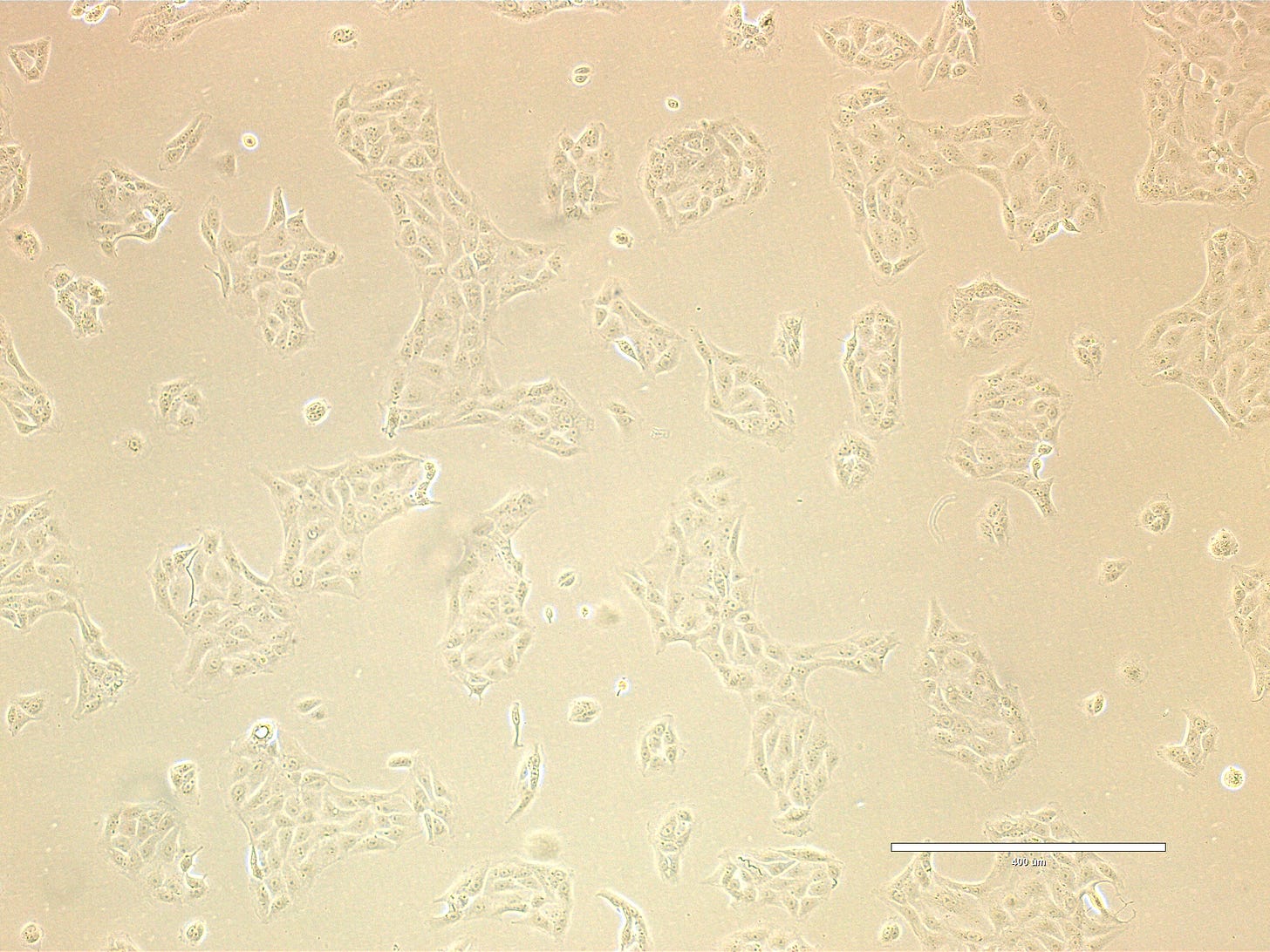
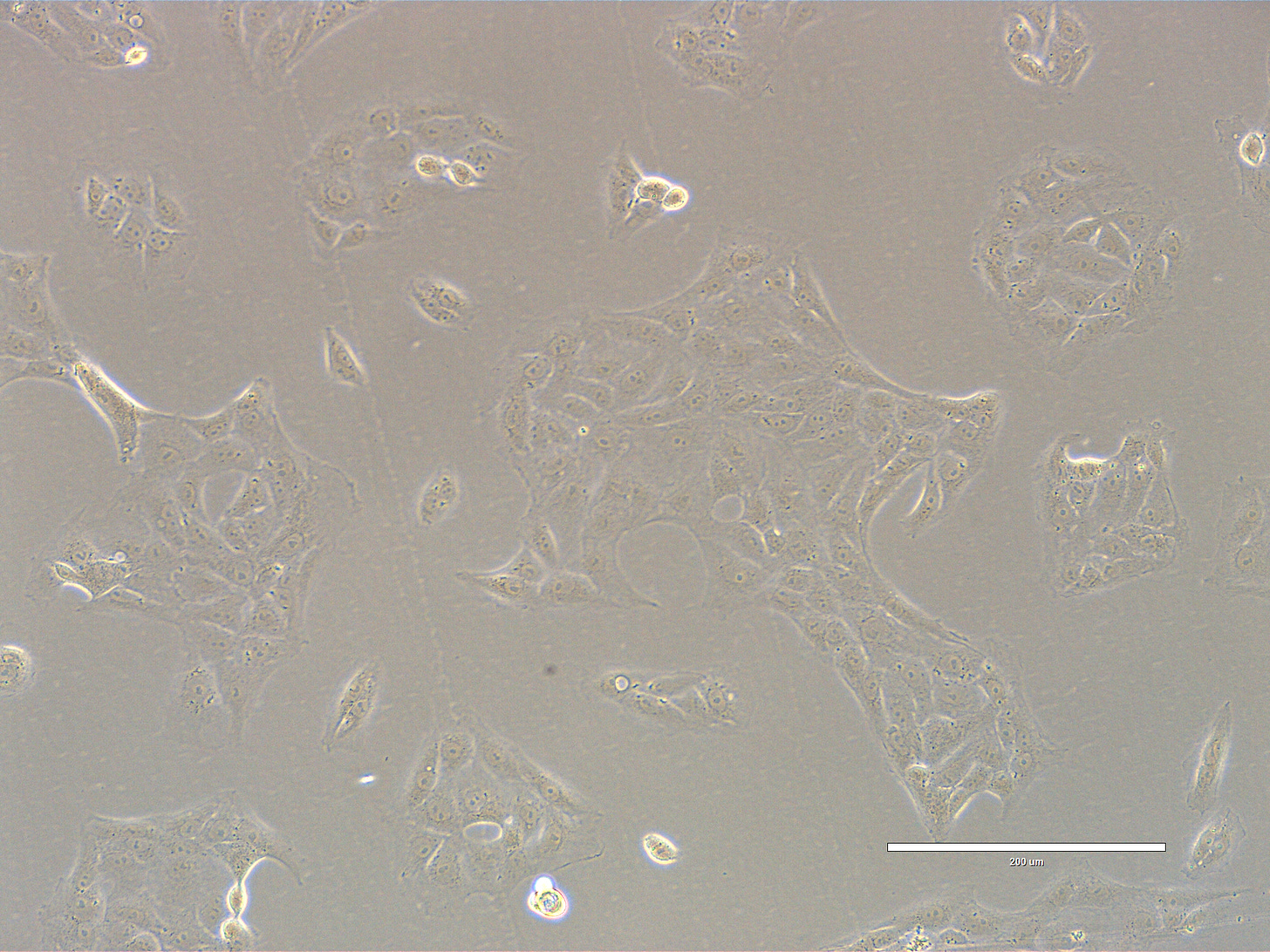
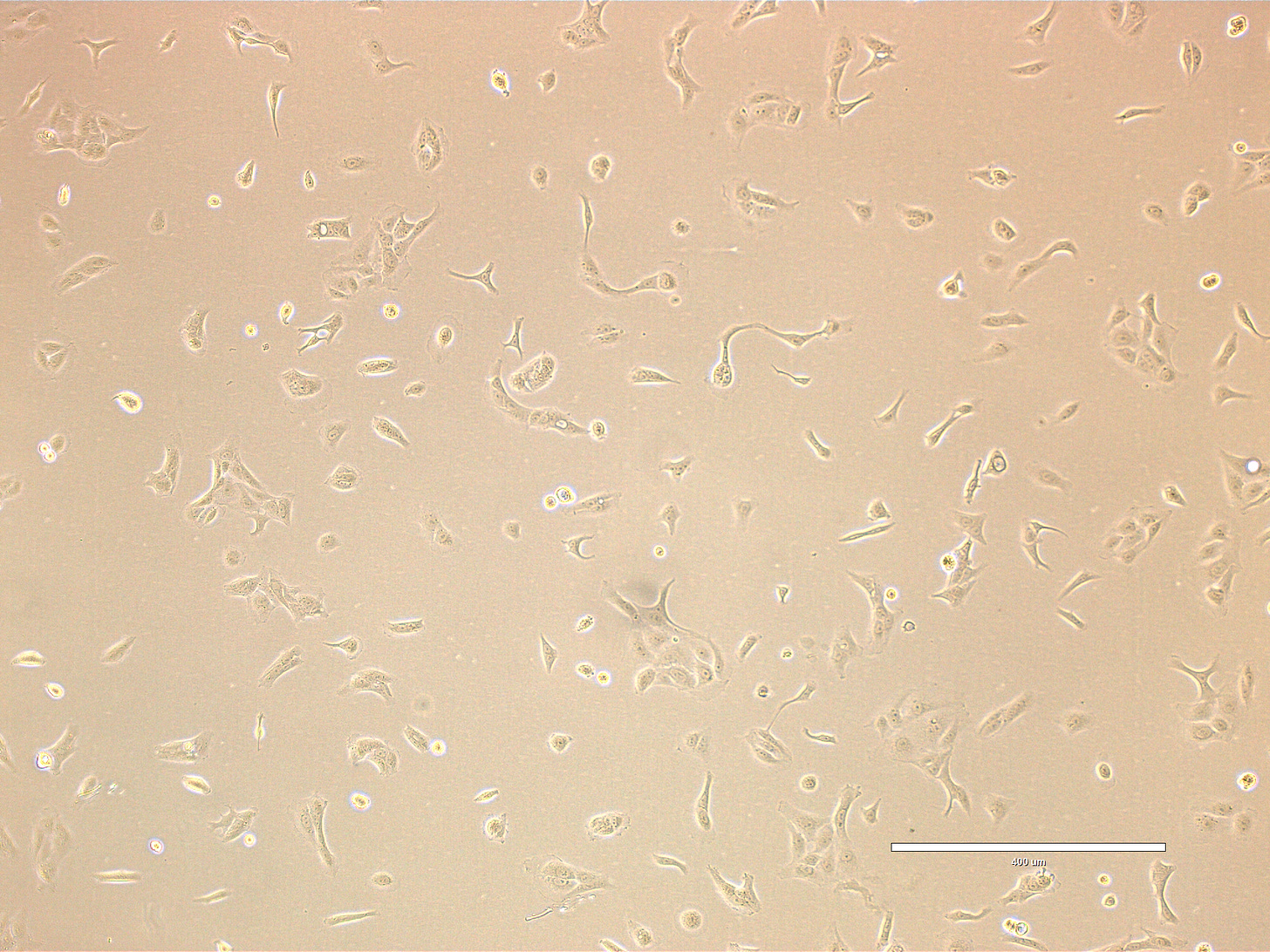
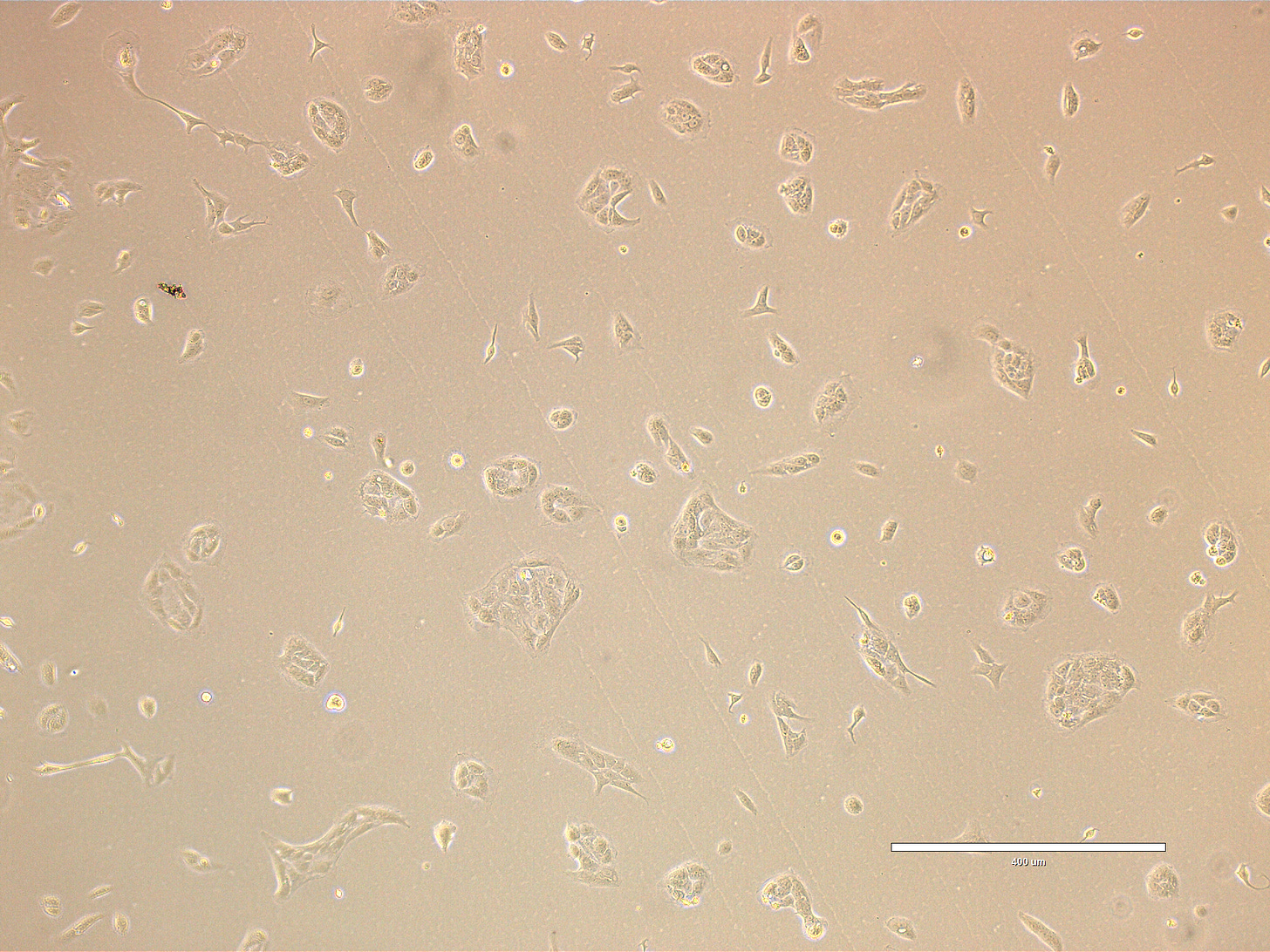
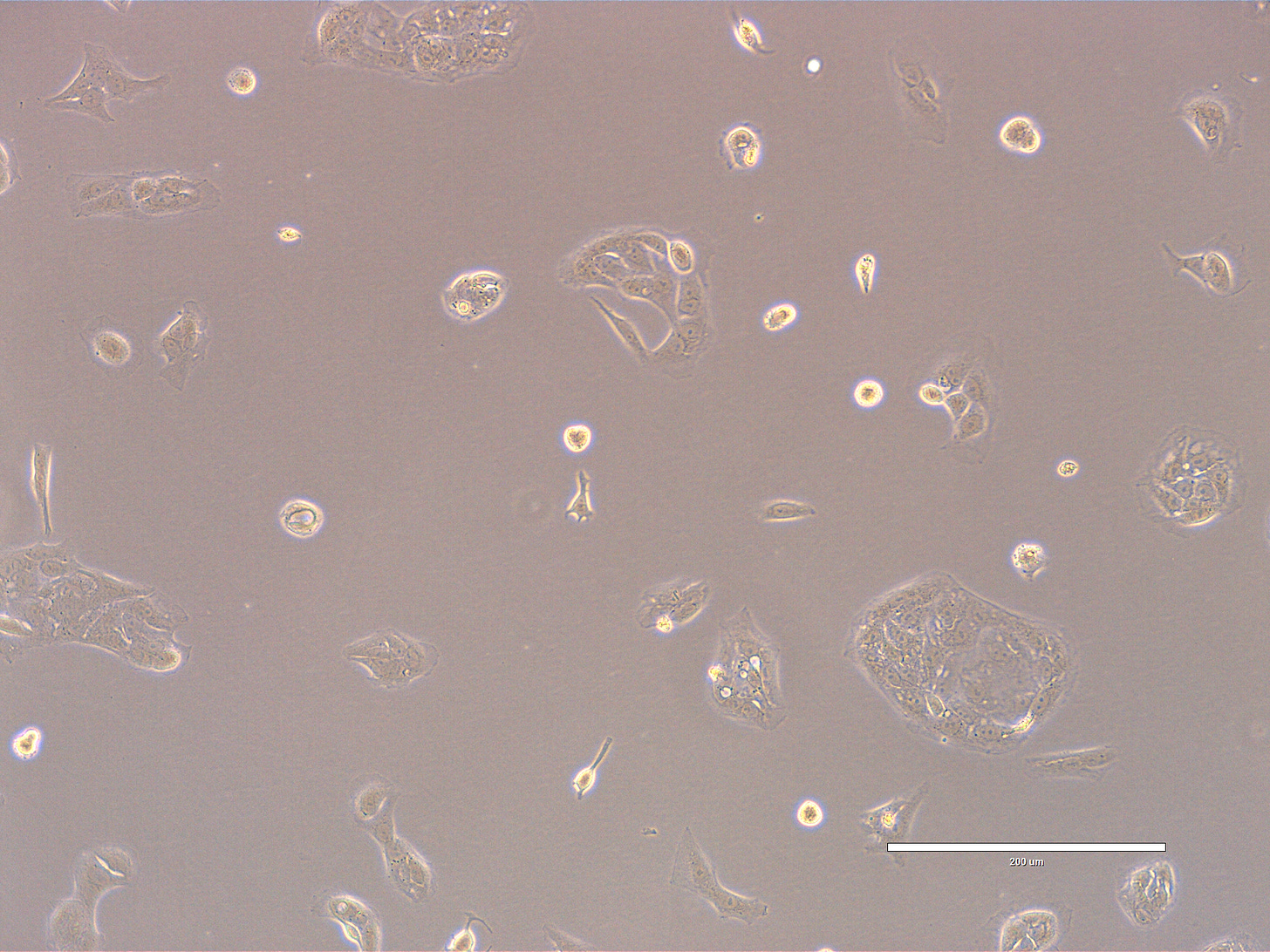
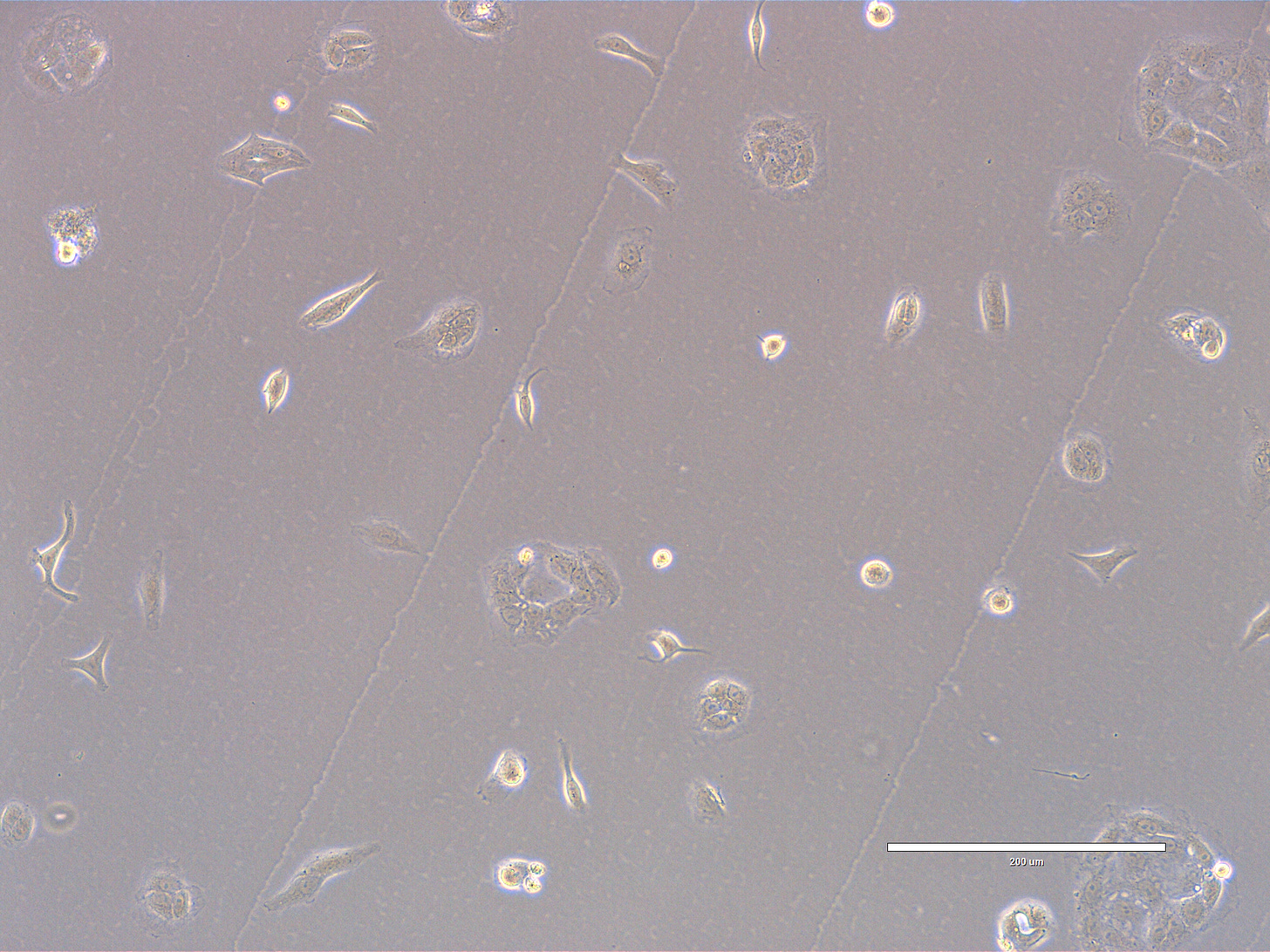
CRO Labs Description of the Cell Cultures
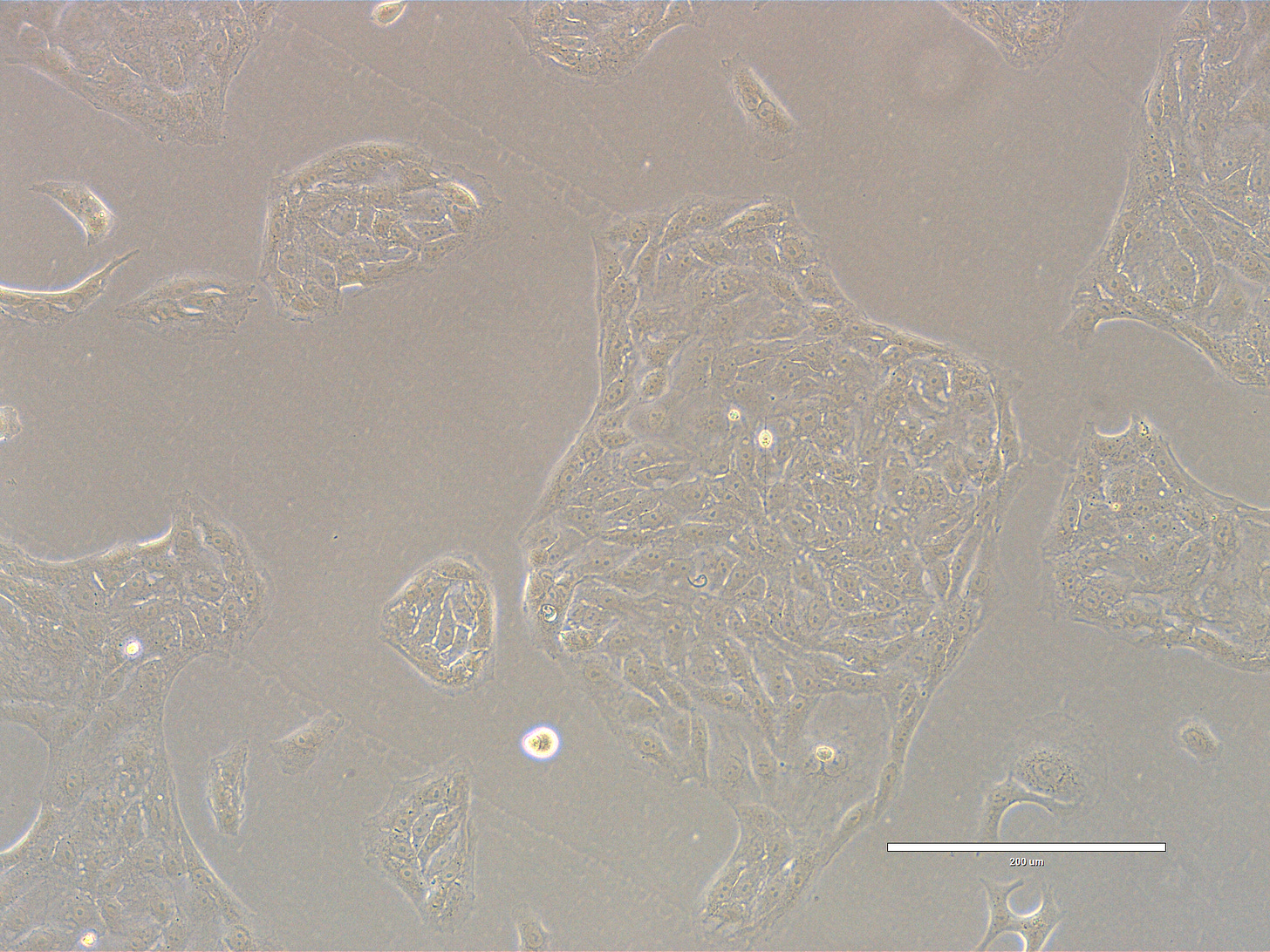
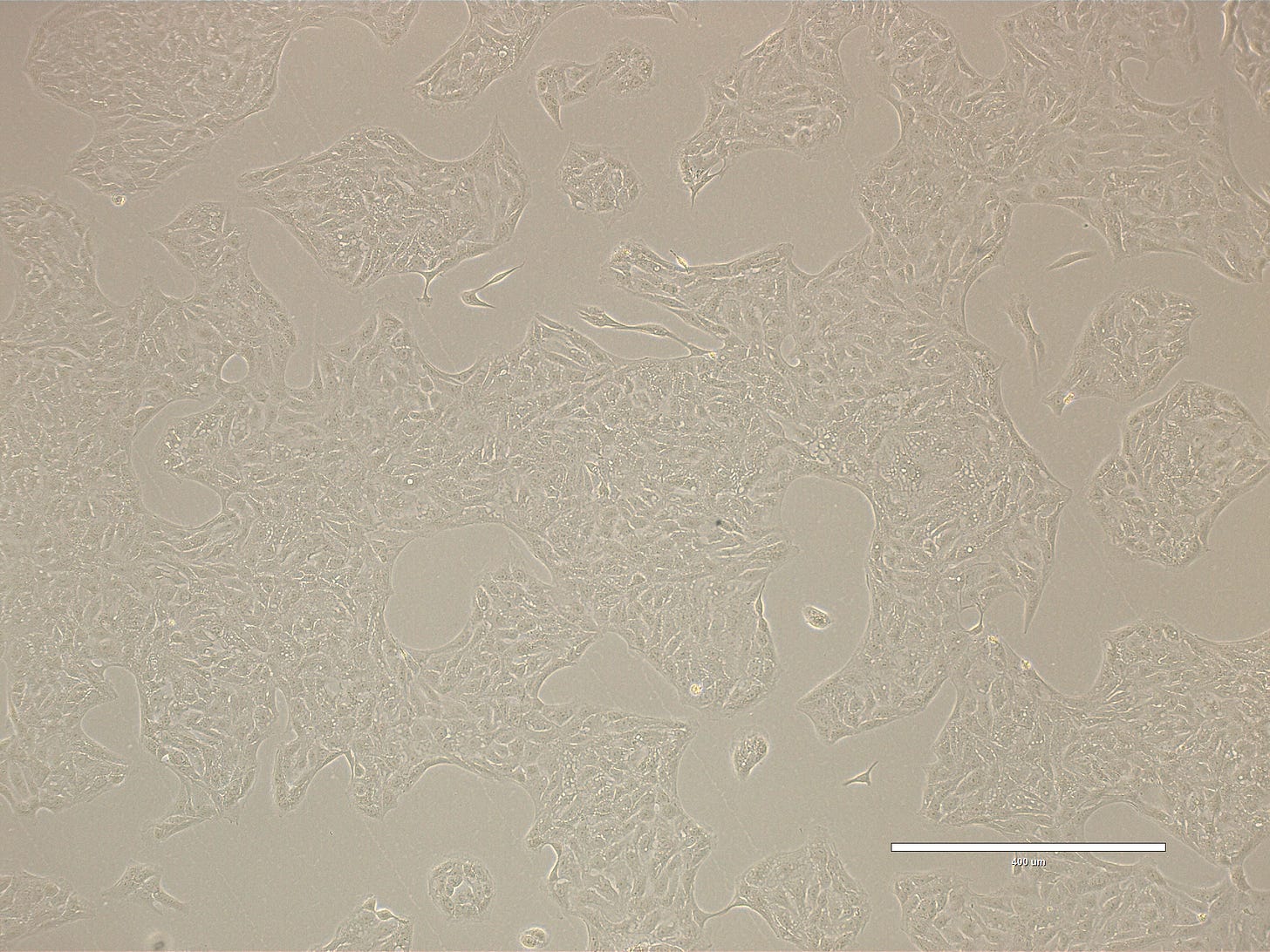
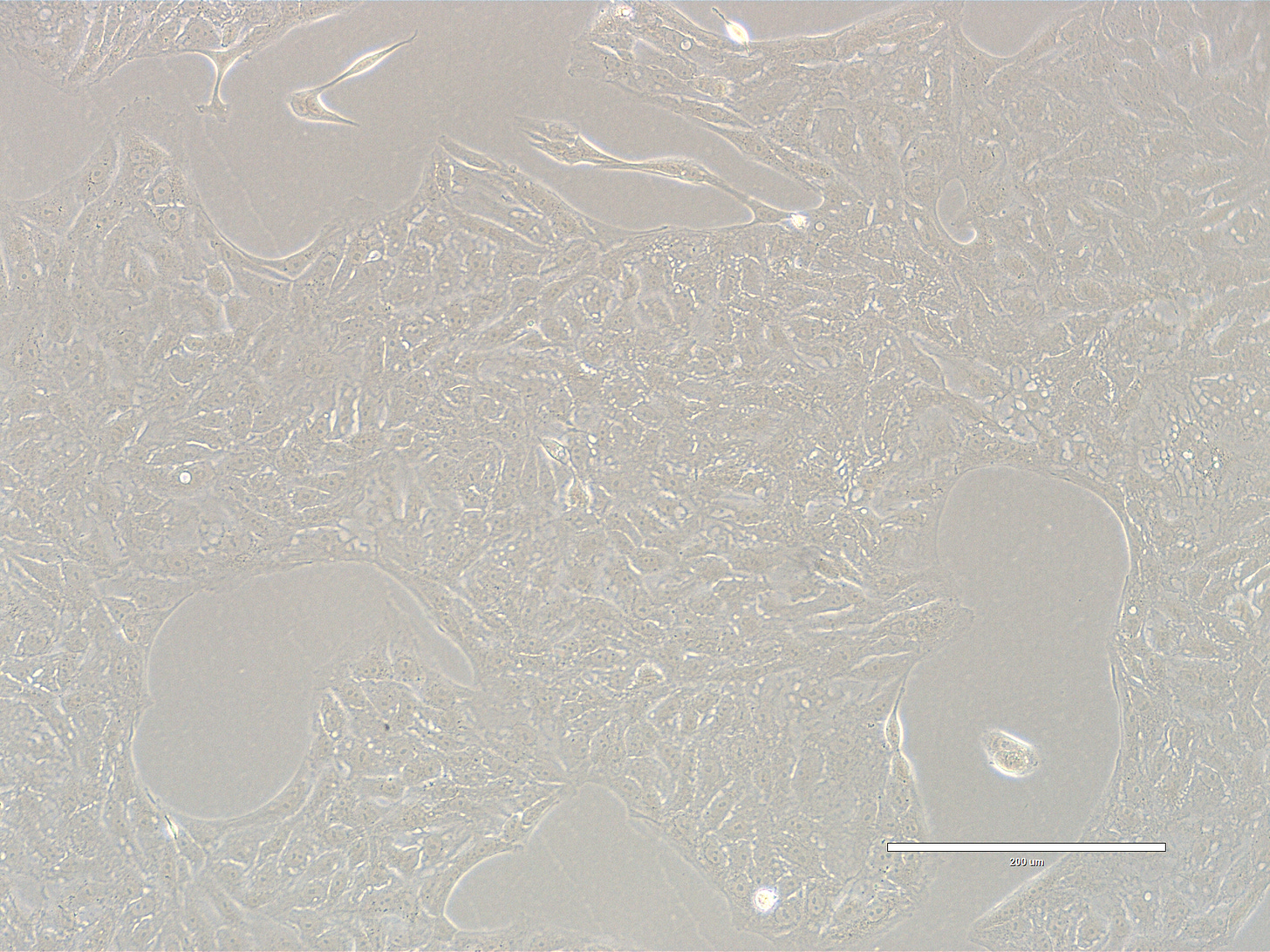
Images are obtained using an EVOS FL microscope using Bright Field or Phase and 10x (400 µm scale) or 20x objective (200 µm scale)
P1 Day 0:
PREP_path1_passage 1_01 to 06
Images of Vero cells taken on day 0 (approximately 1 h after thawing). Individual cells or small clumps consisting of few cells, still in suspension (not attached) are evenly spread out in the images. Cells are bright and rounded, indicating that they are viable. No other morphological features are visible at this point. Bright field. The scale bar is indicative of the objective used.
P1 Day 1:
PREP_path1_passage1_10-19
Images of Vero cells taken 1 day after thawing. Most cells are adherent and display a cobblestone-like morphology, characteristic of epithelial cells. They appear well-spread and evenly distributed, but with noticeable gaps between clusters of cells. Individual cells are oval to polygonal in shape, with distinct nuclei visible and nucleoli (20x). There are a few clumps of cells that are still rounded. The cell borders are clearly defined, and no signs of clumping, detachment, or contamination are present, suggesting healthy growth conditions. Brightfield microscopy.
PREP_path1_passage1_10: low confluency area
PREP_path1_passage1_17: high confluency area
P1 Day 2:
PREP_path1_passage1_201-219
Images of Vero cells taken 2 days after thawing. Most cells are adherent and display a cobblestone-like morphology, characteristic of epithelial cells. They appear well-spread and evenly distributed, but with noticeable gaps between clusters of cells. Individual cells are oval to polygonal in shape, with distinct nuclei visible and nucleoli (20x). Bright rounded dividing cells present throughout the culture. The cell borders are clearly defined, and no signs of clumping, detachment, or contamination are present, suggesting healthy growth conditions. Brightfield and phase microscopy.
PREP_path1_passage1_215: Multiple bright dividing cells throughout the field of view. A clump of bright rounded cells is present in the center of the image. Relatively even distribution of cells thought the image, either as small cell clusters or individual cells. Image PREP_path1_passage1_216 is a magnification (20x) of the center area of this field. Phase microscopy.
P1 Day 3:
PREP_path1_passage1_301-319:
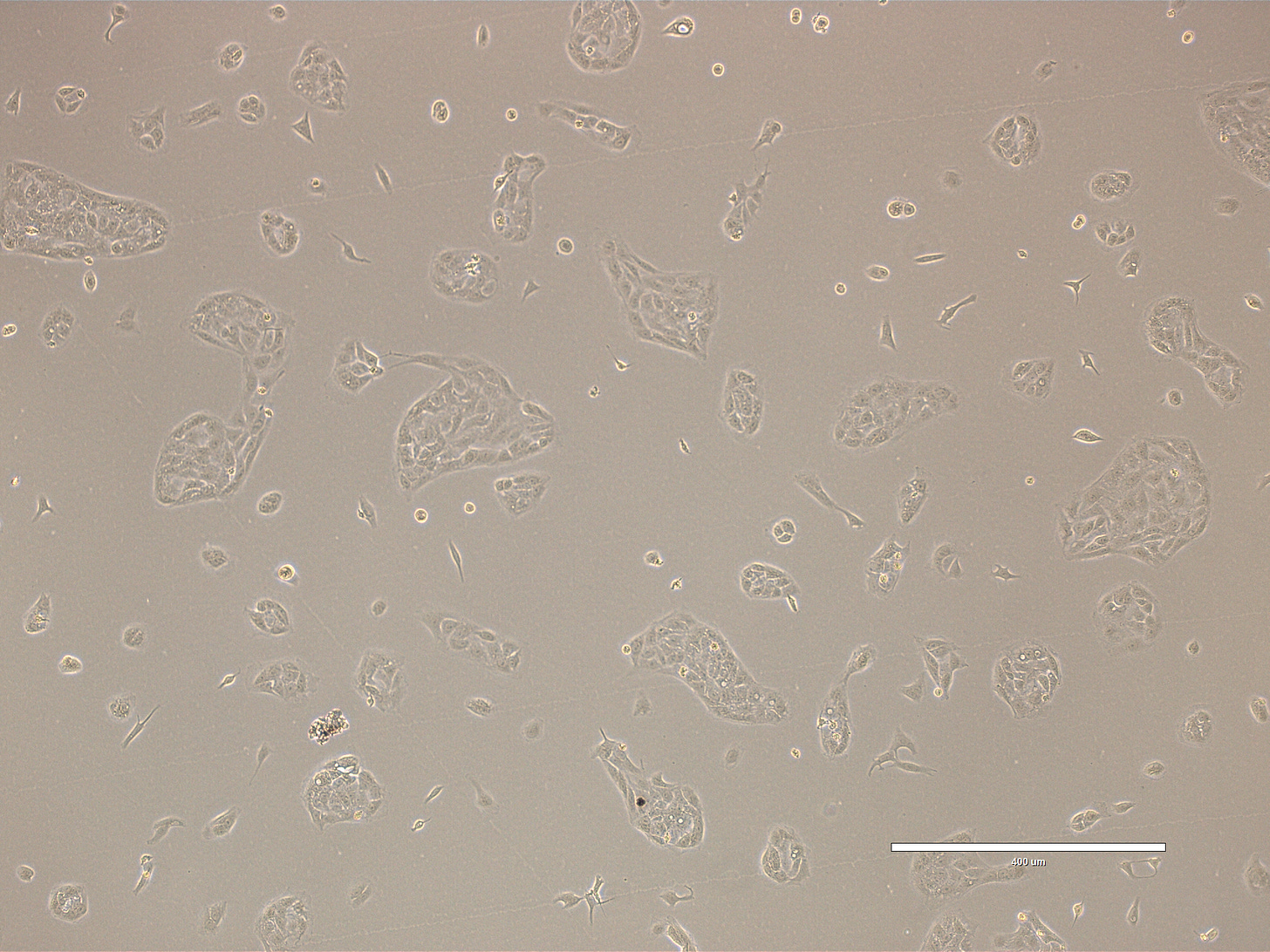
Images of Vero cells taken on day 3 after thawing. The cells are growing in clusters or sheets, with radiating patterns typical of adherent monolayers. Culture is 50-60% confluent with most cells in large cellular islands with large intercellular gaps visible, indicating that the monolayer is not yet complete. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. Bright field and phase.
PREP_path1_passage1_303: There are few rounded dividing cells and some bright reflective points, probably small debris, but no clear evidence of contamination (e.g., bacterial or fungal bodies).
PREP_path1_passage1_317 and 318: High confluency area (~ 80-90%)
PREP_path1_passage1_319: Low confluency area (~ 40%). One dark spot present at 1 o’clock is most likely debris. Dividing bright cells at 7 o’clock.
P1 Day 4:
PREP_path1_passage1_401-413:
Images of Vero cells taken on day 4 after thawing. The cells are growing in sheets that cover most of the surface with few dividing cells present. The cells exhibit a classic polygonal shape, with well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. Bright field and phase.
PREP_path1_passage1_401 and 413: High confluency area (more than 90%) with dividing bright cells and small gaps still visible indicating that this culture needs to be subcultured.
PREP_path1_passage1_411: Lower confluency area
PREP_path1_passage1_412: Large reddish debris in the center of image that is in a different field than the cells.
P2 Day 1:
PREP_path1_passage2_101-113:
Images of Vero cells taken on day 5. The cells are growing in small clusters, with radiating patterns typical of adherent monolayers. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Bright field and phase.
PREP_path1_passage2_102: Small adherent cluster of cells with some mitotic figures in the lower part of the image. Some small bright reflective spot, probably cell debris, present at 12 o’clock.
P2 Day 2:
PREP_path1_passage2_201-216:
Images of Vero cells taken on day 6. The cells are growing in big clusters, with radiating patterns typical of adherent monolayers and large cell-free gaps indicative of a sub-confluent culture. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
PREP_path1_passage2_207: Lower confluency area. Multiple bright dividing cells. PREP_path1_passage2_208: magnified image of the center part of image 207. Center top: cells that underwent karyokinesis (nuclear division) but have not finished cytoplasmic division.
PREP_path1_passage2_208: high confluency area with larger cell clusters
PREP_path1_passage2_213: center- A pair of bright cells observed in mitosis
P2 Day 3:
PREP_path1_passage2_301-312:
Images of Vero cells taken on day 7. The cells are growing in big clusters, with radiating patterns typical of adherent monolayers and large cell-free gaps indicative of a sub-confluent culture. The cells exhibit a classic polygonal or elongated shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or contamination. The scale bar is indicative of the objective used. Phase microscopy.
PREP_path1_passage2_303: center left - A pair of bright cells observed in mitosis
PREP_path1_passage2_305: Some of the cells have cytoplasm with distinct large, round, clear vesicle-like structures. Few bright mitotic figures are present at 2 and 5 o’clock.
P2 Day 4:
PREP_path1_passage2_401-410:
Images of Vero cells taken on day 8. The cells are growing in big clusters covering most of the cell culture surface, with radiating patterns. The cells exhibit a classic polygonal or elongated shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or contamination. The scale bar is indicative of the objective used. Phase microscopy.
PREP_path1_passage2_403 and 409: High confluency areas (~90% confluence). PREP_path1_passage2_404 and 410: magnified image of the 403 and 409 respectively showing little to no space between the cells.
PREP_path1_passage2_405: Low confluency area (~60% confluence).
P3 Day 1:
PREP_path1_passage3_101-110:
Images of Vero cells taken on day 9. The cells are growing in small clusters, with radiating patterns typical of adherent monolayers. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
PREP_path1_passage3_101: Small adherent cluster of cells with some mitotic figures (1 and 11 o’clock).
PREP_path1_passage3_106: Small clusters of cells with radiating patterns. With multiple bright cells (probably undergoing division) and a clear mitotic figure at 4 o’clock (two distinct nuclei, indicating that nuclear division has occurred, but the cytoplasm has not fully divided — this suggests cytokinesis is not complete).
P3 Day 2:
PREP_path1_passage3_201-210:
Images of Vero cells taken on day 10. The cells are growing in clusters, with radiating patterns typical of adherent monolayers. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
PREP_path1_passage3_201: conjoining clusters of cells with some mitotic figures (11 o’clock). PREP_path1_passage3_202: magnification of previous image (top left) showing multiple dividing cells.
PREP_path1_passage3_209: Low confluence area with smaller cluster of cells surrounded by cell free areas.
P3 Day 3:
PREP_path1_passage3_301-310:
Images of Vero cells taken on day 11. The cells are growing in big clusters, with radiating patterns typical of adherent monolayers. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
PREP_path1_passage3_301: conjoining clusters of cells with cell free areas indicating that the culture is not confluent.
PREP_path1_passage3_302: Large cluster of cells covering the surface with some dividing (bright cells) at 1 and 7
PREP_path1_passage3_303 and 304: High confluency area. Cells growing in sheets covering most of the surface area.
PREP_path1_passage3_307: Low confluency area.
P3 Day 4:
PREP_path1_passage3_401-410:
Images of Vero cells taken on day 12. The cells are growing in big clusters, with radiating patterns typical of adherent monolayers covering most of the surface indicating that the culture is ready for sub-culture. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
PREP_path1_passage3_401: cells are growing in large sheets or clusters of cells. Cell-free areas are still present indicating that the culture is not confluent. Very few bright (dividing) cells are present. PREP_path1_passage3_402: magnification of bottom left part of 401 image.
EXPERIMENT P4 Day 1:
EXP_path1_passage4_101-110:
Images of Vero cells taken on day 13. The cells are in small clusters, with radiating patterns. Multiple individual cells and rounded cells dividing cells. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
EXP_path1_passage4_101: most cells are adherent and are growing in small clusters. Multiple bright cells throughout the field.
EXP_path1_passage4_103: Lower confluency area with clusters consisting of less than 10 cells evenly covering the area and multiple bright cells with some mitotic figures in the top left part of the image. EXP_path1_passage4_104: magnification of 103 with a mitotic figure at 3 o’clock.
EXP_path2_passage4_101-110:
Images of Vero cells taken on day 13. The cells display a variety of morphologies, but most appear elongated or spindle-shaped with some degree of spreading, characteristic of fibroblast-like or mesenchymal cells. A few cells appear rounded, which might indicate mitotic activity, poor adhesion, or early signs of stress. The overall confluency is low—likely around 20–30%, meaning the culture is still in early growth phase and has significant space to expand. The cells are distributed evenly across the field, with occasional small clusters. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
EXP_path2_passage4_101: Few small clusters present. Most cells appear elongated or spindle-shaped with some degree of spreading. Multiple cells appear rounded, with some undergoing mitosis (center).
EXPERIMENT P4 Day 2:
EXP_path1_passage4_201-210:
Images of Vero cells taken on day 14. The cells are in small clusters, with radiating patterns. Multiple individual cells and rounded cells dividing cells. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
EXP_path1_passage4_201: Most cells are adherent and are growing in small clusters. Cells undergoing division: image center and 12 o’clock. EXP_path1_passage4_202: Magnification of the center of 101 image with cells undergoing division in the center of the field.
EXP_path2_passage4_201-210:
Images of Vero cells taken on day 14. The cells display a variety of morphologies, but most appear elongated or spindle-shaped with some degree of spreading, characteristic of fibroblast-like or mesenchymal cells. A few cells appear rounded, which might indicate mitotic activity, poor adhesion, or early signs of stress. The overall confluency is low. The cells are distributed evenly across the field, with occasional small clusters. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
EXP_path2_passage4_201: Few small clusters present (less than 10 cells). Multiple single cells with some degree of spreading. EXP_path2_passage4_202: Magnification of the center of 201 image. Small cluster of cells present. Cells appear smaller in size with dense cytoplasm. Multiple cells appear rounded. Some cells have bright reflective points.
EXPERIMENT P4 Day 3:
EXP_path1_passage4_301-310:
Images of Vero cells taken on day 15. The cells are in clusters, with radiating patterns. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
EXP_path1_passage4_305: Cells are adherent and are growing in clusters that are beginning to merge.
EXP_path2_passage4_301-310, 301a-310a:
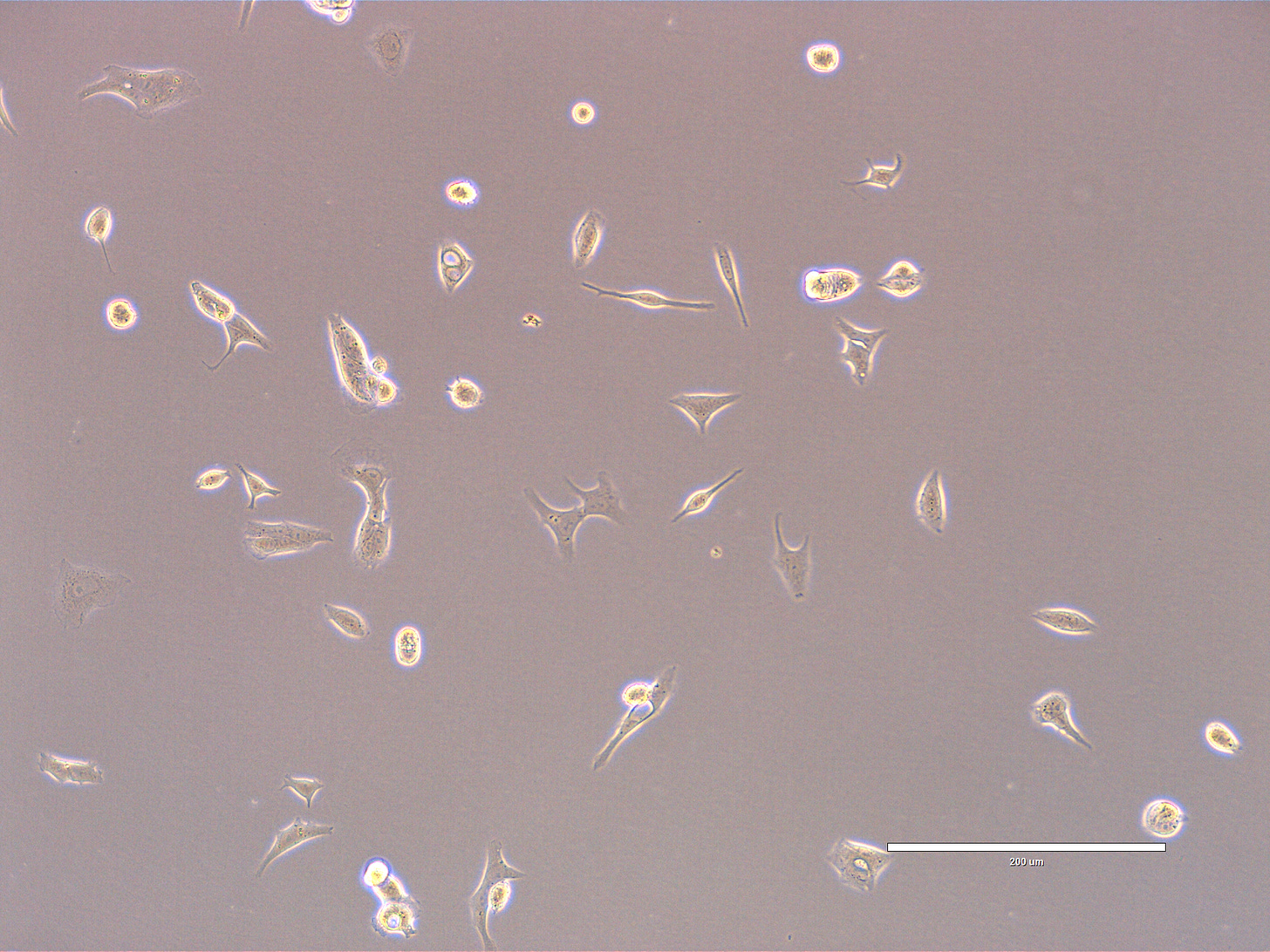
Images of Vero cells taken on day 15. The cells display a variety of morphologies. Few cells are rounded, some with extensions denoting that they are still attached. The overall confluency is low with multiple small clusters and numerous single cells. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment. Cytoplasm appears dense/granular. There are some bright reflective points: some look like large vacuoles whereas some are cellular debris. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
EXP_path2_passage4_303: Few small clusters present with some bright spots (vacuoles). There is a pair of dividing cells at 7 o’clock. Multiple single cells with some degree of spreading. Multiple cells appear rounded.
EXP_path2_passage4_307 and 308: Low confluency area and very small clusters. Multiple single cells evenly spread out in the field, some with cytoplasmic extensions.
EXPERIMENT P4 Day 4:
EXP_path1_passage4_401-410:
Images of Vero cells taken on day 16. The cells are in large coalescent clusters, with radiating patterns. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
EXP_path1_passage4_401: Cells are adherent and are growing in clusters that are beginning to merge. Very few bright dividing cells. A mitotic figure close to the bottom left corner of the field.
EXP_path1_passage4_406: Large clusters of cells that begin to merge covering ~ 50% of the surface. Some cells have clear bright vesicles.
EXP_path2_passage4_401-410:
Images of Vero cells taken on day 16. The cells display a variety of morphologies. Few cells are rounded, some with extensions denoting that they are still attached. The overall confluency is low with multiple small clusters and numerous single cells. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment. Cytoplasm appears dense/granular. There are some bright reflective points: some look like large vacuoles whereas some are cellular debris. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
EXP_path2_passage4_402: Few small clusters present with some bright spots (vacuoles). There is a pair of dividing cells at 7 o’clock. Multiple single cells with some degree of spreading. Multiple rounded cells. Some cells appear rounded and refractile, which could indicate potentially dying cells (6 o’clock).
EXP_path2_passage4_405, 406: Low confluency area few very small cell clusters. Multiple single cells evenly spread out in the field, some with cytoplasmic extensions. Some round cells have granular and irregular shape, most likely dying cells or debris.
EXPERIMENT P4 Day 5:
EXP_path1_passage4_501-510:
Images of Vero cells taken on day 17. Large coalescent clusters or cells with radiating patterns and few bright dividing cells. The cells exhibit a classic polygonal or elongated, fibroblast-like shape, with visible cytoplasmic extensions and well-defined nuclei. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment or cytoplasmic granulation. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
EXP_path1_passage4_501: Cells are adherent and are growing in large coalescing clusters. There are cell free areas throughout the field denoting that culture did not reached confluency.
EXP_path2_passage4_501-510:
Images of Vero cells taken on day 17. The cells display a variety of morphologies. Few cells are rounded, some with extensions denoting that they are still attached. The overall confluency is low with multiple small clusters and numerous single cells. The cells look healthy, with intact membranes, good attachment to the substrate, and no obvious signs of detachment. Cytoplasm appears dense/granular. There are some bright reflective points: some look like large vacuoles whereas some are cellular debris. The scale bar is indicative of the objective used. No clear evidence of contamination. Phase microscopy.
EXP_path2_passage4_507, 508: Low confluency area few very small cell clusters. Multiple single cells evenly spread out in the field, some with cytoplasmic extensions. Some round cells have granular and irregular shape, most likely dying cells or debris.
ANALYSIS AND INTERPRETATION
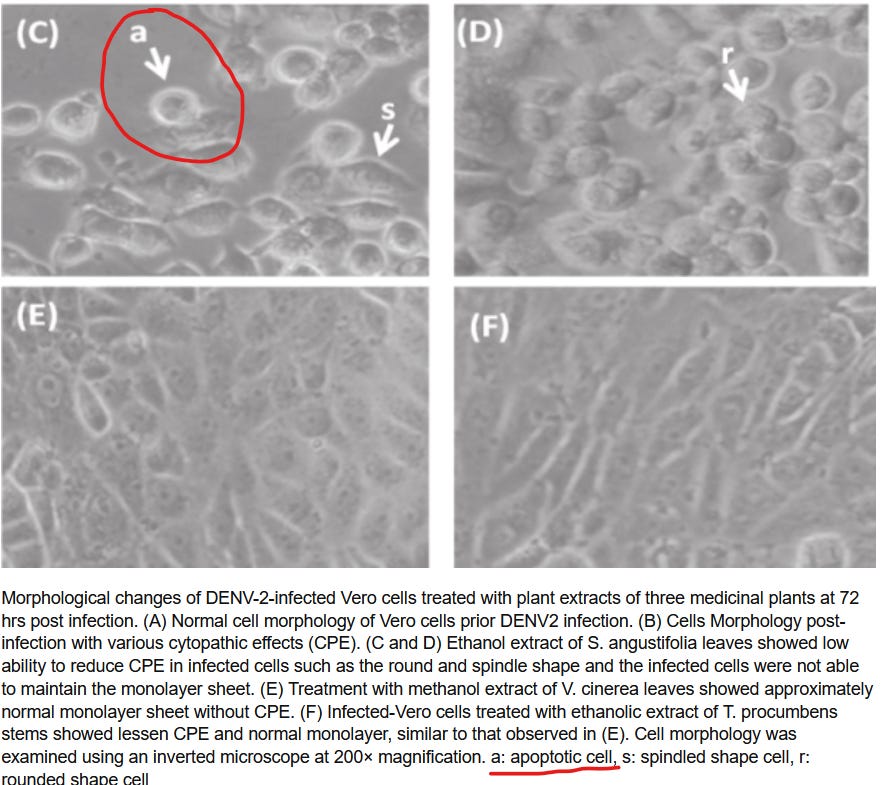
Outlined here are the exact, claimed to be specific, Cytopathic Effect Morphologies that are seen in the cells in standard Virological Infections and their descriptions. With each morphology, examples of images from Published virological infections will be shown besides images of the Experiment Plates and Descriptions from the Contract Lab Technician whom carried them out. Where there is an image, it will link to the publication in which it came from:
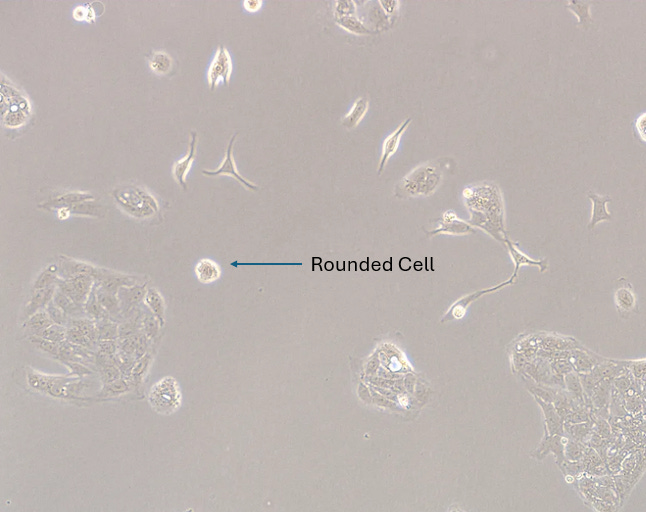
1. ROUNDING
Published References
A:
B.
Experiment
CRO Description:
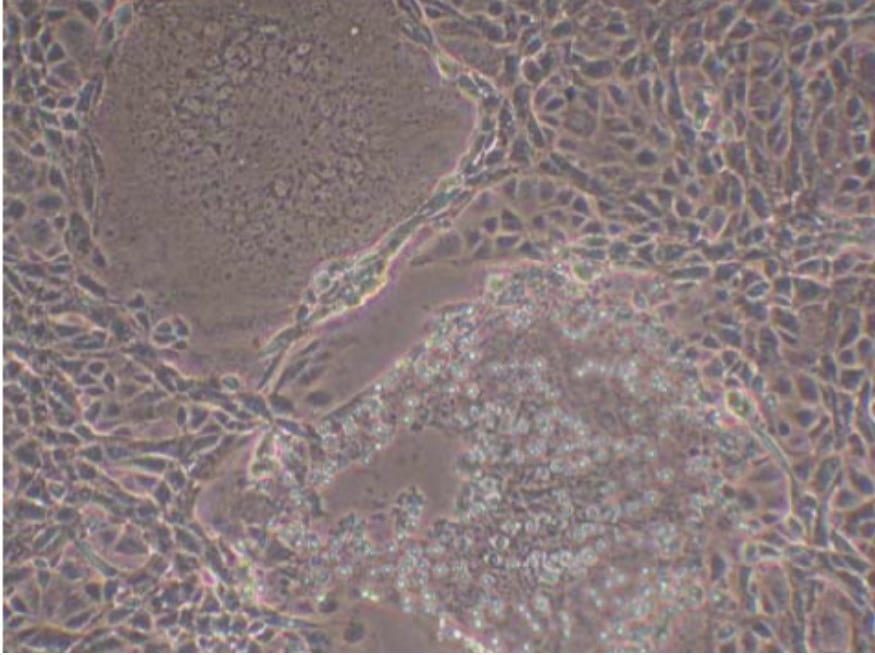
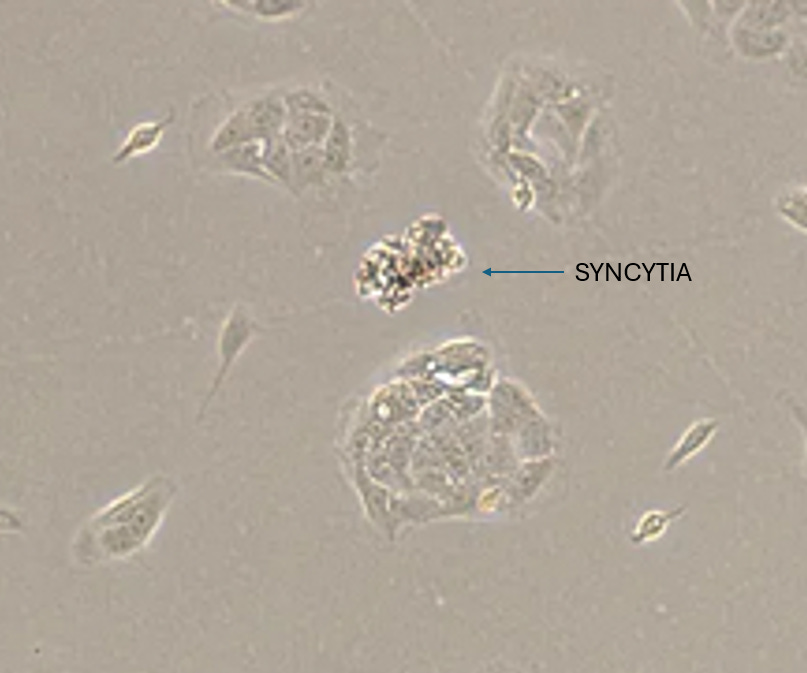
2. SYNCYTIA
Published References
Experiment
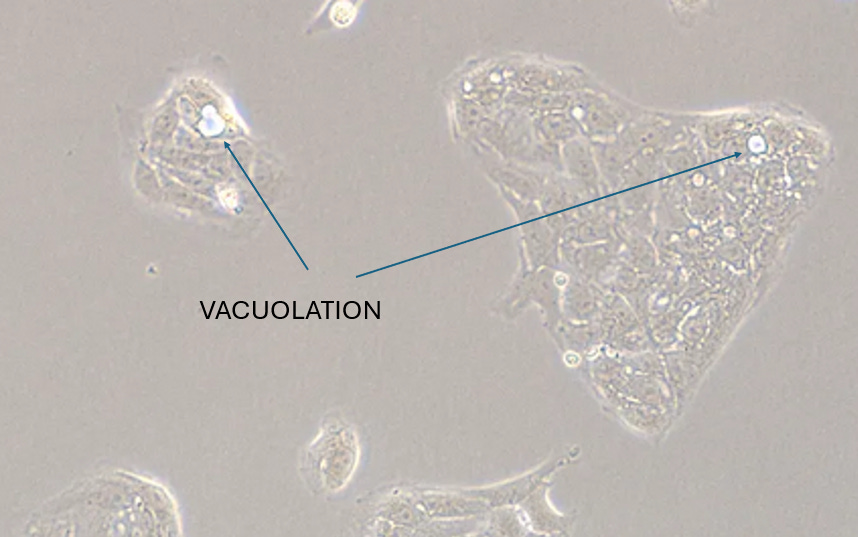
3. VACUOLATION
Published References
A:
B:
Experiment
CRO Description
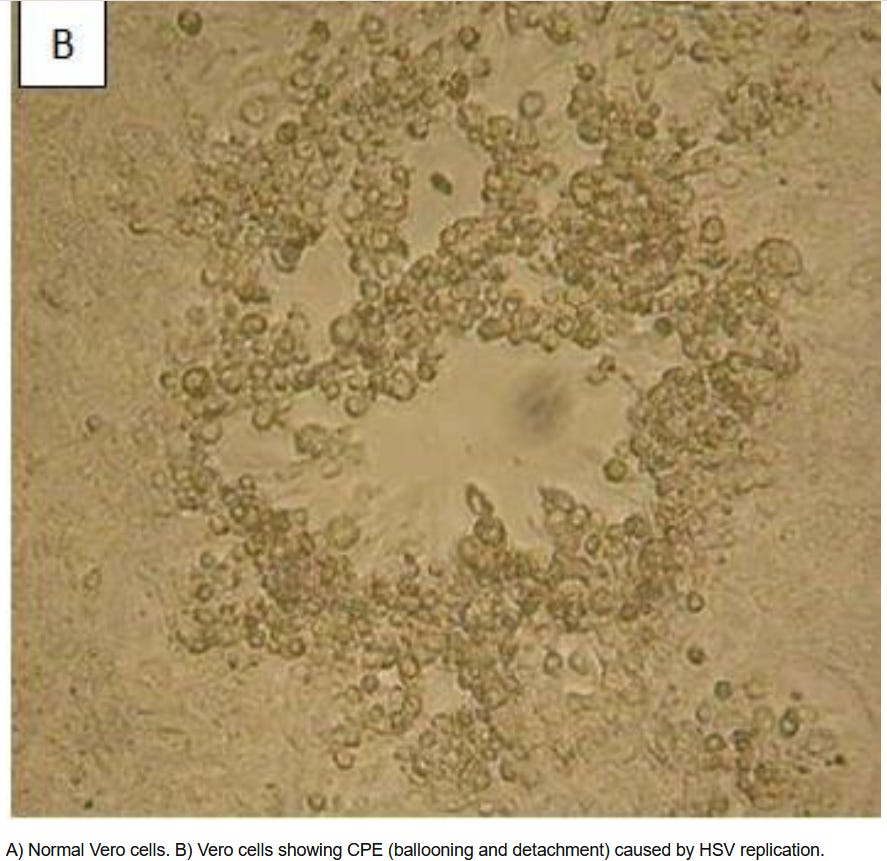
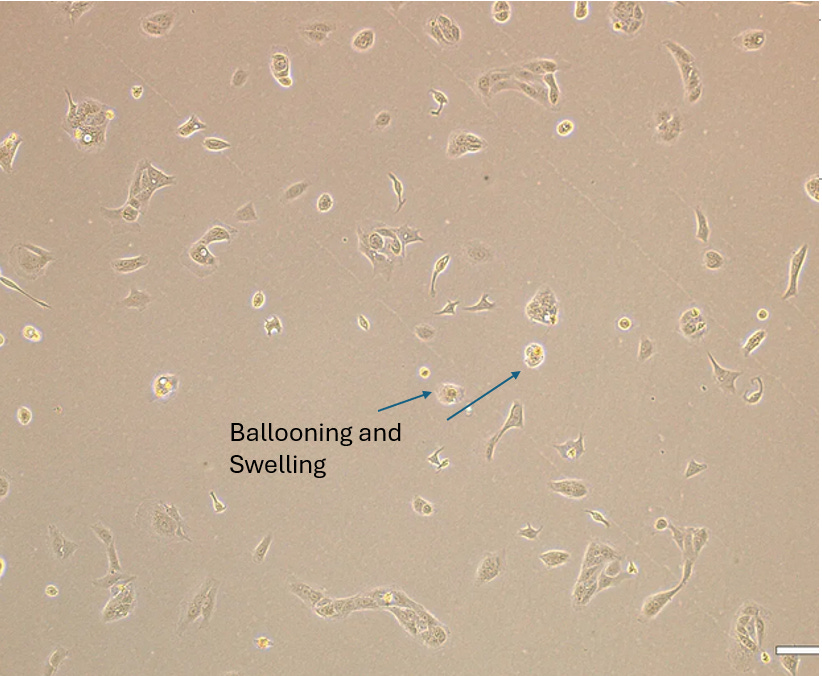
4. BALLOONING AND SWELLING
Published References
Experiment
5. DETACHMENT OF MONOLAYER
Published References
Experiment
CRO Description
6. Plaque Formation
Published References
Experiment
CRO Description
7. Cytoplasmic Inclusions
Published References
Experiment
CRO Description
8 & 9 Necrosis and Apoptosis
Published Reference
Experiment
CRO Description
CONCLUSION
The Agreed upon reference material to outline protocol in Virological Isolation in Cell Cultures is defined by the Clinical & Laboratory Standards Institute (CLSI) in the reference manual titled M41:
“This CLSI guideline provides essential guidance for viral culture and identification using commercially available cell cultures and reagents in clinical virology laboratories. It outlines critical factors for reliable viral culture, including cell culture selection, maintenance, quality control, specimen preparation, isolate identification, and result interpretation.”
In this reference manual it clearly instructs that all infection mediums should lower FBS concentration well below the 10% growth medium, down to a suggested 1-3% FBS:
Here the American Society for Microbiology states that any CPE morphologies seen in culture prior to 5 days incubation is enough to meet the threshold to indicate Viral Presence:
Here AI is agreeing with the American Society for MicroBiology that the threshold of criteria to meet for a Cell Culture to be infected with a virus is the noting of a single CPE morphology:
The Independent Laboratory whom carried out the experiment were not aware of the aims of this study, they were briefed that the Cell Culture protocol was to starve the Cell Line, so that it may produce Extra Cellular Vesicles that we would study under Transmission Electron Microscopy. Despite being blinded to the aims of the study, they verified in the description of the Cultures 6 out of 9 of the CPE morphologies said to indicate viral presence.
The CPE morphologies that the Laboratory Technician didn’t explicitly note in the Culture were involved with broader morpholiges of multiple cells such as Synctyia and Plaque Formation. Although these morphologies were present in the culture they were not prevalent, certainly not in the same manner as were seen in the first Study with the HEK cell lines.
The confluence in the first study on Day 1 of incubation was ~90% , there were still pockets allowing growth and the DMEM remained red showing that the cells were not starved through overcrowding. In contrast, this study started both paths at a low confluence ~30%, the growth medium Path 1 grew with a finishing Confluence of ~80%. The low FBS Path 2 exhibited stagnation of the cell line and apoptosis, finishing at day 5 with a low confluence ~15%, noted by the CRO. If the Confluence had started at a higher percentage as in the first study it would seem more likely conducive to the broader CPE morphologies like Synctyia and Plaque Formation as seen in the first.
Given the clear instruction of the Clinical & Laboratory Standards Institute to reduce the FBS concentration on infection and the threshold of viral presence being any CPE Morphology to be observed. The results of this study that verify our initial findings clearly demonstrate that this Serum reduction is the cause of claimed CPE and claimed Viral presence in the culture.
It is clear from these comprehensive studies that the Gold Standard in viral Isolation is not a valid method and renders the downstream BioChemical assays used to indicate Viral presence as invalid given that no Purified or Isolated distinct Biological particle has been produced to benchmark against as a Gold Standard.
























Somehow... I am not surprised. Thank You for all You do to show the farce of virology.
Great work Jamie,
Yet another study that can be added to the list showing that CPE is non specific.
https://zenodo.org/records/15872332