CELL CULTURE ISOLATION CONTROL EXPERIMENT
All of the content that is put out on this Substack is going to be for free. If you feel so inclined to donate or Sign up for a Paid Subscription that is very much appreciated. It will keep me writing, putting out content and continuing the largest Control Studies Project falsifying Virology.
HYPOTHESIS:
Uninfected Cultures with reduced FBS concentration environments and increased Antibiotic concentration that directly match the concentrations of infection mediums in the cell culture viral isolation procedure, produce the observable morphological changes of Cytopathic Effect in the cell line, to the same extent and in the same time as infected cultures.
Materials and Methods
Cell Culture
Human Embryonic Kidney (HEK293T ATCC CRL-3216) cells were seeded onto 6-well culture dishes at passage 5-8 at a concentration of 1x106 per 5mL and grown for 3 to 4 days at 37⁰C, 5 % CO2 in a humidified incubator. Conditions were as follows:
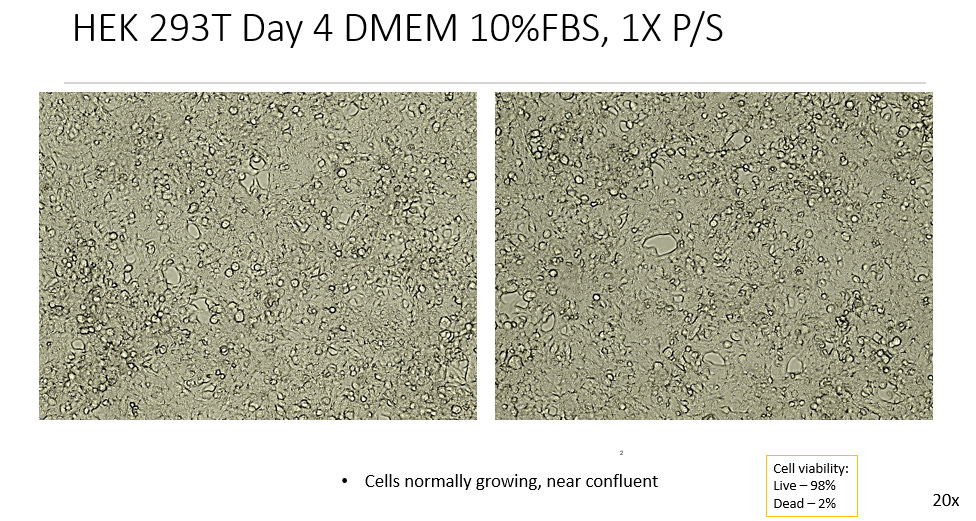
1. DMEM, 10% FBS, 1x P/S
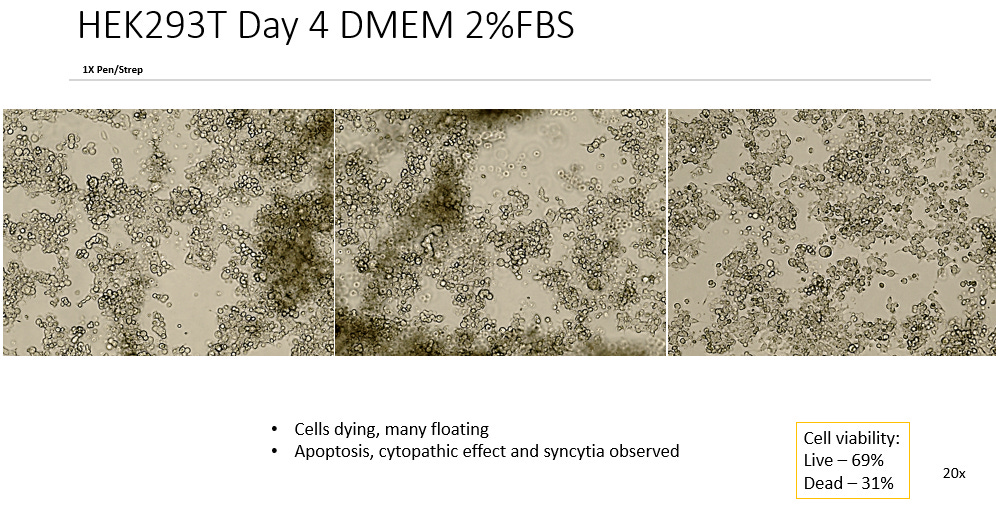
2. DMEM, 2% FBS, 1x P/S
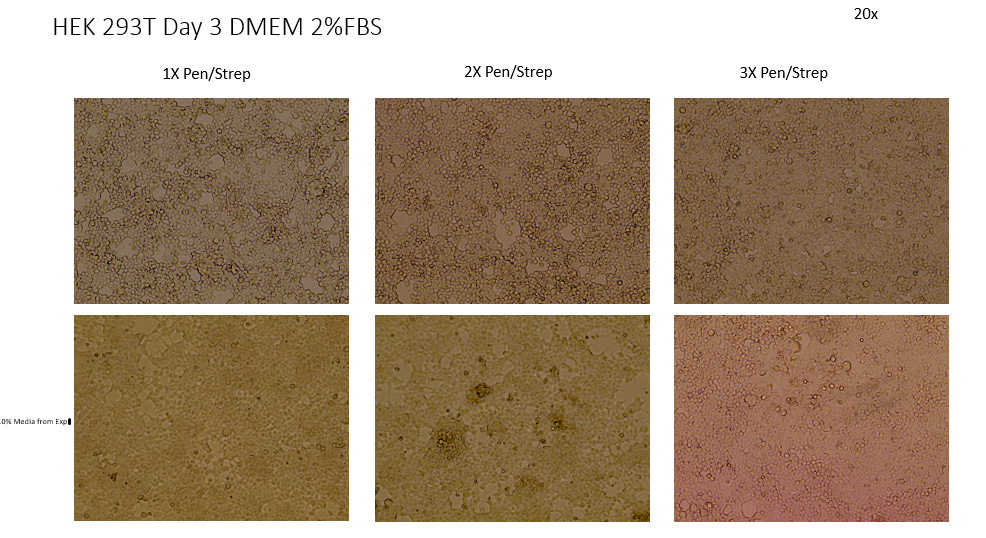
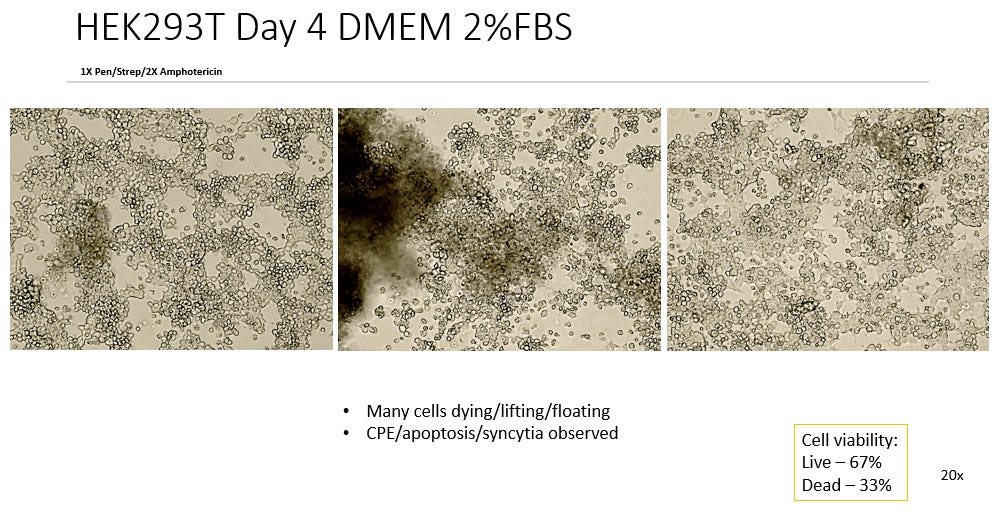
3. DMEM, 2% FBS, 2x P/S
4. DMEM, 2% FBS, 3x P/S
5. DMEM, 1% FBS, 1x P/S
6. DMEM, 1% FBS, 2x P/S
7. DMEM, 1% FBS, 3x P/S
At day 3 or day 4 of growth, cells were imaged at 20x with an Evos XL Core Imaging System.
After imaging cells, cultures were washed with PBS, then 1 mL of TryplE was added to dissociate cells from the dishes. After 3 minutes, 5mL of DMEM+10%FBS+1xP/S was added and cells were harvested then centrifuged at 1000 x rpm for 3 minutes. The cell pellets were re-suspended with 5mL of DMEM+10%FBS+1x P/S and viability was calculated by Countess 3 FC Automated Cell Counter (Invitrogen).
After viability was recorded, cells were centrifuged at 1000 rpm for 3 minutes, then the DMEM was poured out and the cell pellet was re-suspended in 300-500ul of TRIzol and stored at -80⁰C until further analysis.
Documented Video Method
LABORATORY VIDEO DOCUMENTATION OF THE CELL CULTURE ISOLATION CONTROLS AND METHOD
All of the content that is put out on this Substack is going to be for free. If you feel so inclined to donate or Sign up for a Paid Subscription that is very much appreciated. It will keep me writing, putting out content and continuing the largest Control Studies Project falsifying Virology.
Test Results
Experiment 1
Negative Control:
Test:
Experiment 2
Negative Control + 10% Media from Exp 1
Test
Experiment 3
Negative Control
Test
Experiment 4
Negative Control
Test
Experiment 5
Negative Control
Test
Experiment 6
Negative Control
Test
Experiment 7
Negative Control
Test
Experiment 8
Negative Control
Test
Experiment 9
Negative Control
Test
ANALYSIS OF RESULTS
Ending with a confluence between 85-99%, the Negative Controls remained healthy, alive and with space to grow, so that no apoptosis or breakdown of the cell line was observed. Past day 5 of incubation it may have become difficult to maintain that level of confluence, running the risk of the cell line starting to breakdown due to overgrowing, contact inhibition and starvation. Hence the tests were only done to a maximum of 5 day incubations, despite many protocols of Viral Isolation exceeding this time period.
Comparing the test cultures to the Negative Controls in 10% FBS we see unanimous difference in all 105 test cultures (Some not pictured) irrelevant of slight variations in FBS or Antibiotic Concentration. All test cultures showed apoptosis of the cell line starting as early as 48 hrs into incubation. All test cultures exhibited what can be described as Cytopathic Effect in the cell line with noted distinct morphological features such as Plaque Formation, Ballooning, Rounding, Floating, Lifting and Syncytia.
This observed Cytopathic Effect gradually increased in all cultures over time ranging from ~10%-40% cell death measured by the Countess Cell viability counter. This represents considerable death of the cell line.
A number of verification tests were employed to control for potential limiting factors such as seeding density and Nutrient Verification in the form of DMEM concentration. Seen in the video method, there are 6 plates that were seeded to half the density at 0.5million cells/ 5ml compared to the test cultures. It was noted that on imaging at days 3 and 5 that there was very little difference between the observed CPE in the low density and test plates. This rules out any observed effects off cell death that could be attributed to over growing cells or the cells suffering from any contact inhibition in the test plates.
Also in the video method, the colour of the plates even at the later stages of day 5 of incubation, were noted to be of red colour, indicating a plentiful supply of DMEM still left in the culture. This shows that in standard Virological isolations where DMEM is used as nutrient medium, this is not the contributing factor to cell death observed over time.
There was a slight increase in CPE observed between the test plates of 1% FBS and 2% FBS. The difference was more pronounced in the earlier stages with observed CPE differing to a max of 10% at day 3. In all cultures the difference was negligible at day 5. In some protocols of viral isolation where 1%FBS is used in infection medium and the cultures are only incubated for short periods of time <72hrs, this could be seen as a determining factor, but overall is not as marked as the initial starvation from growth (10%) to infection medium (2%).
With increasing concentrations of antibiotics and addition of Amphotericin in some plates having negligible effect to the CPE observed. In a couple of plates notably in Experiment 4/2% the 3x Pen/Strep jumps by 10% CPE, however this may have been a potential misread by the Countess as the image of the cell line looks similar to to the others in plate.
In Experiment 2, 10% of the culture medium was used to “infect” the culture. This was to look at the type of Plaque Assay such as TCID₅₀ in serial dilution. Despite only one dilution being performed there was a noted change in the cell line that slightly increased the observed CPE and also introduced some distinct morphological CPE features such as Syncytia seen most notably in Day 3 and 5 2%. If this dilution were to be serial it is is implied that those formations and observed CPE would become increasingly more apparent, although further research is needed in this area.
A human sample was provided from a healthy individual to act as a test to compare standard viral isolation protocols in the later experiments. This introduced another source of cells into the line which were noticeably different when imaged and seemed to somewhat disrupt the line, however did not change the amount of CPE or morphologies observed in any meaningful way.
There were a few papers that were used as protocol for the experiments that were cross-referenced to act as a Positive Control. Although the findings in this experiment were not reliant on a benchmark of other protocols, the results by imaging comparison showed that a strong positive indication was received in the test cultures of the experiment, in some cases even exceeding that of the appearance of the Positive Control adding weight to our findings.
This can be seen here.
The Positive Control
All of the content that is put out on this Substack is going to be for free. If you feel so inclined to donate or Sign up for a Paid Subscription that is very much appreciated. It will keep me writing, putting out content and continuing the largest Control Studies Project falsifying Virology.
Conclusion
The unanimous results in the breakdown of the Cell Line in the test cultures occurred at the same speed, to the same severity and included all of the distinguishing morphological features of CPE observed in the cross referenced papers. According to The American Society of Microbiology any CPE observed in <5days of incubation is indicative of viral presence and replication.
The protocols of all viral isolation in the infection medium, all incubate cultures in reduced FBS environments. This is claimed to be what is described as a “Maintenance Medium” implying that a cell line would be maintained in a stable form over the entire course of the incubation, thus demonstrating that all of the observed effects of CPE and Morphological features were solely caused by the contents of the infected sample.
The strong evidence shown in this paper heavily suggests that the reduced FBS environments are actually the cause of the observed effects of CPE and Morphological features and hence the claimed infected sample cannot be determined to be a contributing factor whilst using this method.
This would further suggest that claimed isolated and replicated viruses assumed to be harvested from the cell culture isolation method should not be considered legitimate as the claimed observable effects that indicate the presence of a virus are present in uninfected cultures.





































There is no other way to better demonstrate the unscientific nature of virology than this, I think.
If supposedly "infected" cell cultures show the same result as cell cultures in which no patient sample was used, that is the end of the chain of evidence of virology.
The "virological method" is fundamentally inapplicable, as this would require the existence of an uninfected patient sample and an infected patient sample. However, neither exists, and even if they did, they would not be distinguishable from each other. In virology, the chronology of events is reversed, in a crazy feedback loop. Effect Y causes X, the trigger .
You would first have to use suitable isolates to determine whether infected and non-infected material exists, and isolation without cell culture and the inevitable effect of CPE does not exist according to virology. Virology goes round in circles around a centre that cannot be presented in a logical and solvable way. A self-fulfilling prophecy.
Anyone who wants to should continue to believe it and bathe in their hysterical fear. But the chain of evidence is not enough for subjugation and violation of human rights. Full stop, the end!
Jamie, thank you. Every cent is well spent.
Thanks for the detailed explanation and documentation. In short: virologists kill healthy cells in the lab by starving the cells to death. Virologists are cell killers. We expect the PCR controls to further strengthen the evidence.
Thank you Jamie and your team.